Durability of Geotextiles (Educational Research)
Submitted by Xinli Liu, Rammohan Nanjundappa, and Unchin Cho
Introduction
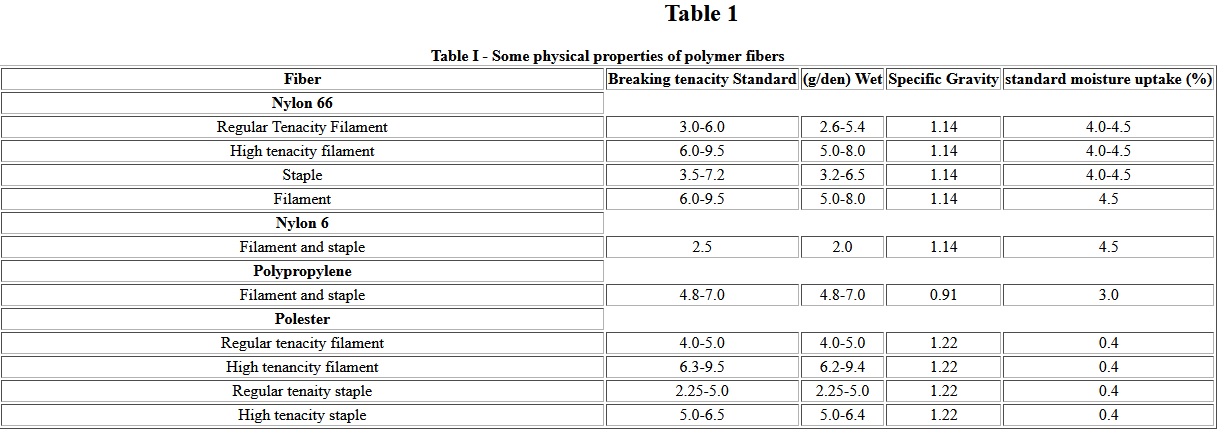
Geosynthetics have become increasingly important in the construction and environmental industries. Included in the general geosynthetic classification are: geotextiles, geomembranes, geonets, geogrids and geocomposites . A variety of polymers including polyolefins, polyesters, nylons, and polyvinyl chloride are currently being used as geosynthetics. The development of synthetic Fibers after 1939 and of nonwoven manufacturing processes in the mid-1960s have revolutionized textile usage in civil engineering practice. Even though woven fabrics are used as geotextiles due to their higher tenacities and high modulus, about 70% geotextiles are nonwoven fabrics because of higher permeability, better friction, better conformability and construction survivability. Table I summarized typical properties of these polymer Fibers used in the constructions of geotextiles [2].
Table I
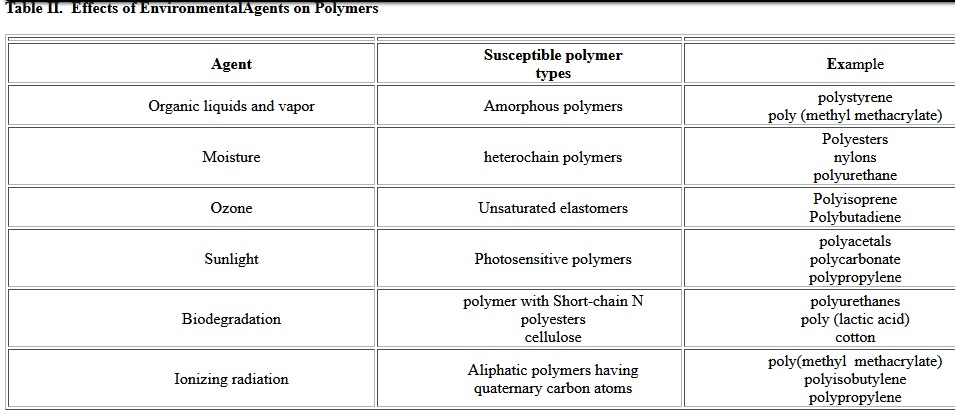
The durability of geotextiles strongly depends on material degradation. This is influenced by the inherent properties of Fibers, such as their chemical nature and properties controlled by processing variables. The practical consequences of degradation are discoloration, surface crazing (formation of surface micro-cracks), embrittlement, and loss of mechanical properties (elongation, impact strength, tensile strength). Considering applications of geosynthetics are almost always outdoors, the degradation of polymers must be taken account when select polymers. There are many environmental factors which influence aging mechanisms of geotextiles. Polymers can degrade by exposure to high temperature (thermal degradation), oxygen and ozone, ultraviolet light, moisture, radiation and chemical agents. Often multiple exposures, such as a combination of moisture and heat or oxygen and light, can result in accelerated deterioration. Deterioration of plastics under normal environmental conditions is called weathering[3]. Table II shows how different polymers respond to different environmental agents.
Table II
Basically, we can categorize the factors which influence polymer durability into two types of degradation processes. The most important for polymer materials is based on the chemical degradation including photo- and thermo- oxidation, hydrolysis, and degradation by chemicals. The other is based on the biological degradation (degradation by microorganisms). The latter plays a less important role in polymers because most polymers are fairly stable to microorganisms.
Polyolefins are chemically stable polymers. They have very good processibility and a low cost compared to other raw materials used in geotextiles. The disadvantages of polyolefins are their sensibilities to high temperature and UV radiation. Therefore, they are subject to thermal oxidation and to photodegradation. By comparison, nylons have good chemical hydrolysis resistance under normal conditions, and they are more resistant to thermo- and photo-degradation than polyolefins. However, they are easily attacked by strong mineral acids, oxidizing agents and some salts [2] and stabilizers such as copper salts with amines are generally required for increasing durability. In the case of polyesters, such as PET, there is high resistance to thermal and photochemical oxidation, but stabilizers such as triphenyl phosphate are generally added to increase the oxidation resistance. PET has high resistance toward various types of chemicals, but it may be degraded under strong acidic and alkaline conditions, due to the hydrolysis of the ester groups.
Overall, polypropylene is the polymer of choice for use in many of these applications due to its unique physical properties, excellent durability, chemical resistance, and low cost [4, 5]. More than 80% geosynthetic products are made from polypropylene [1].
In this paper, we briefly discuss the mechanisms of degradation and examine the most used polymers in geotextiles (polypropylene and polyesters), in terms of their chemical and biological stability in application.
Mechanisms of Degradation
The theory of polymer aging and stabilization is a branch of chemical kinetics, the science of chemical reaction rates [6]. The mechanism of degradation of many polymeric materials is generally thought to proceed via a radical chain pathway [7]. One good example is polypropylene.
Polypropylene is susceptible to oxidation, particularly at elevated temperatures during exposure to ultraviolet light. Oxidation usually leads to increasing brittleness and deterioration in strength. Generally, the mechanism of oxidative degradation is free radical and is initiated by the thermal or photolytic cleavage of bonds. The free radicals then react with oxygen to yield peroxides and hydroperoxides [3]. Additionally, the presence of reactive impurities such as residual catalyst or chromophoric groups can aid in radical initiation. Propagation and termination steps during polymerization probably involve a reaction with oxygen, which can result in the formation of hydroperoxides. Subsequent decomposition of these unstable hydroperoxides lead to further production of reactive radicals. The effect of this degradation process on the physical characteristics of geosynthetics is of critical importance. Degradation of polyolefins results in two opposing effects on molecular weight distribution. Chain scission (decreased molecular weight) and crosslinking (increased molecular weight) may both occur during aging. Polyethylene typically experiences less chain scission than crosslinking, thus, the melt flow rate is usually observed to decrease, reflecting an overall increase in molecular weight. Conversely, polypropylene undergoes chain scission which results in an increased melt flow rate.
Thermal degradation can also cause chain scission reactions involving
the breakage of backbone bonds to yield free-radical segments such as
polypropylene. Nonchain scission effects involving the elimination of
small molecule from a substituent group and double-bond formation such as
poly(vinyl chloride) can also occur. Chain scission can occur by one of
three mechanisms [3]:
- random degradation, where the chain is broken at random sites,
polypropylene degrades in this manner.
- depolymerization, where monomer units are released at an active
chain end
- weak-link degradation, where the chain breaks at the lowest-energy bonds
The chain scission reaction of polypropylene is illustrated as following [4]:
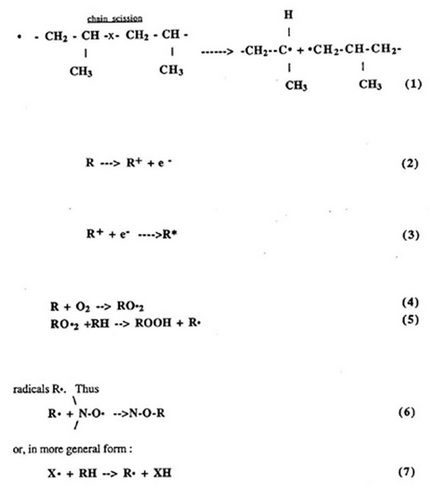
In addition to thermal energy, degradation may be initiated by photochemical action, irradiation, or mechanical action. Photodegradation basically is caused by electromagnetic radiation where the amount of energy absorbed by a molecule must exceed the bond energy to cause degradation [8]. Depending on the energy absorbed by the molecule, the electrons are promoted from a ground state to a higher energy state level, i. e., excited state. Depending on chemical structure, the energy absorbed can be transferred, emitted, dissipated and/or can cause chemical conversion. Some polymers are very radiation resistant (such as polystyrene and polysulfone), but polypropylene will readily degrade upon irradiation. The primary event in radiation damage is the ejection of a high-energy electron:
formula
This primary electron can then ionize additional molecules with the release of additional electrons in a chain reaction. An electronically excited state will result when a positively-charged molecule is recombined with an available electron:
formula
The excited state results in the formation of radical species, which can react with oxygen in the atmosphere, causing chain scission [3].
Polymer degradation can also result from mechanodegradation, which is caused by the application of stress such as high shear deformation. The stress-induced degradation may result from comminution (grinding, milling, or crushing), stretching, fatigue, tear abrasion, or wear. Nonetheless polypropylene has good chemical and hydrolytic resistance.
Mechanism of Stabilization
Mechanisms of Oxidation Stabilization [6]
There are many methods available to hinder the oxidation of polymers. These methods incorporate the choice of the optimum processing conditions, control of the chemical and physical structure of polymeric material, and the addition of special agents, known as antioxidants or oxidation inhibitors.
One way to reduce polymer oxidation is to retard the rates under which the degeneratedly branched chain reacts to oxygen. The steps are: initiation, propagation, branching and termination of the chain.
In initiation step retarding oxidation is done basically by purifying reactants and removing impurities, and deoxygenating the polymer by manufacturing polymer under inert gas.
At the chain propagation step, reduced oxidation is accomplished by using compounds which react with radicals Ro or ROo. The chain propagation step of oxidation reaction includes two basic subreactions:
formula
An effective method of thermal stabilization is through the use of a radical terminating antioxidant. Stable nitroxyl radicals, for example, can terminate the chain by reaction with radicals Ro. Thus or, in more general form :
formula
where RH is polymer, X is antioxidant. The rate constants of reaction (5) are 10-100 times lower than those of reactions between alkyl radicals and oxygen. To compete with chain transfer (reaction 4) the polymer must contain 10-100 times as much antioxidant as oxygen.
A number of stable nitroxyl radicals have been studied as antioxidative additives for polypropylene. In the presence of stable radicals, the induction period of polypropylene oxidation increases. The results show that the best antioxidant is 2,2'-methylene-bis(4-methyl-6-tert.butylphenol).
Another class of compounds capable of inhibiting oxidation through reactions with alkyl radicals are quinones. Both stable radicals and quinones apparently may be used as antioxidants under conditions of oxygen deficiency.
The concentration of RO2o radicals in an oxidized polymer is many times greater than radicals, and RO2o are more reactive in relation to phenols and aromatic amines than Ro. The reaction between phenol or amine and a peroxide radical can be presented as:
formula
where IH is either phenol or amine. For this reaction to result in chain termination, the radicals Io formed by this reaction must have a low reactivity in relation to the oxidized compound RH. These radicals must leave the reaction system through processes which do not result in any new chains.
The most common class of antioxidant for radical termination is a hindered phenol (9). The mechanism, shown in Figure 1, by which these compounds function is by abstraction of H from the hindered phenol by the reactive peroxy-radical. This produces a less reactive, resonance stabilized phenolic radical. Peroxycyclohexadienones can then be formed after reaction with a second peroxy-radial (10). This type of stabilization is effective at temperatures encountered during both melt processing and long term heat aging.
Figure 1 - sorry, the image file is no longer available.
The oxidation inhibition at the chain-branching step is achieved with the help of compounds reacting with hydroperoxides or hydroperoxide groups with a low or zero yield of free radicals. The primary valence-saturated product of polymer oxidation is the hydroperoxide groups formed on the macromolecules. The hydroperoxides decompose by a bimolecular reaction
formula
Since the free radicals produced in hydroperoxide decomposition initiate new oxidation chains (the formation and subsequent decomposition of hydroperoxide groups is in fact a chain-branching process), then their decay resulting in the absence of free radicals will decrease the overall oxidation rate. Sulfur compounds, such as sulphoxides (dioxide) and dilauryl thiodipropionate, are most frequently used in this area. Some phosphorous derivatives including trialkyl phosphites and mixed fatty aromatic phosphites, also are used to reduce hydroperoxides and peroxides. The reaction is characterized by a low free radical yield, resulting in the formation of ion-radical pairs:
formula
Aromatic phosphates can also terminate the oxidation chains according to the reaction:
formula
Hydroperoxides and peroxides can also react with amines:
formula
Phosphites and thioethers (11-14) are effective at decomposing hydroperoxides into stable non-radical products. As previously discussed these peroxy moieties are thermally and photolytically unstable and typically decompose, producing two radical products. Interrupting this process through the use of phosphites or thioethers can significantly reduce the level of radical initiation. Phosphites are very effective during melt processing (typical temperature range: 220-315 oC), providing color and melt flow rate stability. Thioethers (typically used in combination with hindered phenols) are used for elevated temperature (>100oC) applications.
There are other mechanisms such as increasing the quadratic chain termination rate to retard oxidation, or using free radical precursors.
Mechanisms of Light Stabilization
Absorption of radiation basically is an energy absorption problem and it is frequency dependent as shown in Planck's law: E=hv. The principle of photodegradation is the energy absorbed by a molecule has exceeded the bond energy, resulting in photoreaction, and degradation. Depending on the conditions, the primary photochemical reactions that can take place include [8]:
- cleavage into free radicals
- abstraction of hydrogen atom
- photosensitization
- decomposition with formation of two or more molecules
- photodimerization or crosslinking
- intramolecular rearrangement
- photoisomerization
Most polymers, especially polypropylene, are more sensitive to UV than other light sources. Only UV stabilization will be discussed here.
UV stabilizers can generally be categorized into three types. The first class of stabilizers function by screening the polymer through absorption of UV radiation. The stabilizers absorb UV radiation and prevent polymer degradation . BY undergoing a reversible tautomeric process, they dissipate the UV energy as heat. As shown in Figure 2, the UV absorber (UVA) absorbs the radiation and is converted into its keto-tautomer. The most commonly used UV absorbers are substituted benzotriazoles or benzophenones. A disadvantage of UVA's is that their effectiveness is limited by Beer's absorption Law, which requires high concentrations and sufficient polymer thickness. In colored samples, the pigment serves as a screening agent; therefore, the benefit of UVAs are often significantly reduced.
Figure 2
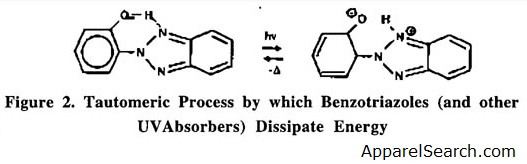
Another class of stabilizers act as UV quenchers or energy transfer agents. These compounds function by quenching molecules that have become excited and returning them to the ground state before homolytic bond cleavage can occur. This class of stabilizers are typically nickel-based coordination complexes.
The most recent class of light stabilizers are the Hindered Amine Light Stabilizers (HALS) which function by interrupting the radical chain degradation mechanism. HALS's stabilization mechanism is shown in Figure 3. The proposed stabilization mechanism involves the formation of a nitroxyl radical from the oxidation of the hindered amine. This nitroxyl radical can, in turn, react with free radicals in the polymer to eventually yield nonradical products. In contrast to the sacrificial mechanism proposed for hindered phenolic antioxidants, it is believed the nitroxyl radicals can be regenerated and thus function in an efficient manner. This may explain the high level of UV stabilization achieved at relatively low concentrations.
Figure 3 - sorry the image file for figure 3 is not longer available.
Factors Affecting Durability of Geotextiles
Chemical degradation
The chemical degradation is greatly dependent on the chemical structure of polymers. Generally speaking, synthetic polymers are remarkably stable under most environmental conditions. However, certain type of polymers can be more sensitive to the certain type of environments. The environmental conditions should be considered for geotextiles are as following:
- gases and vapors, mainly oxygen and water
- acidity and alkalinity of the soil
- other chemicals in the soil
- electromagnetic radiation, especially ultraviolet rays from sunlight
- elevated temperatures
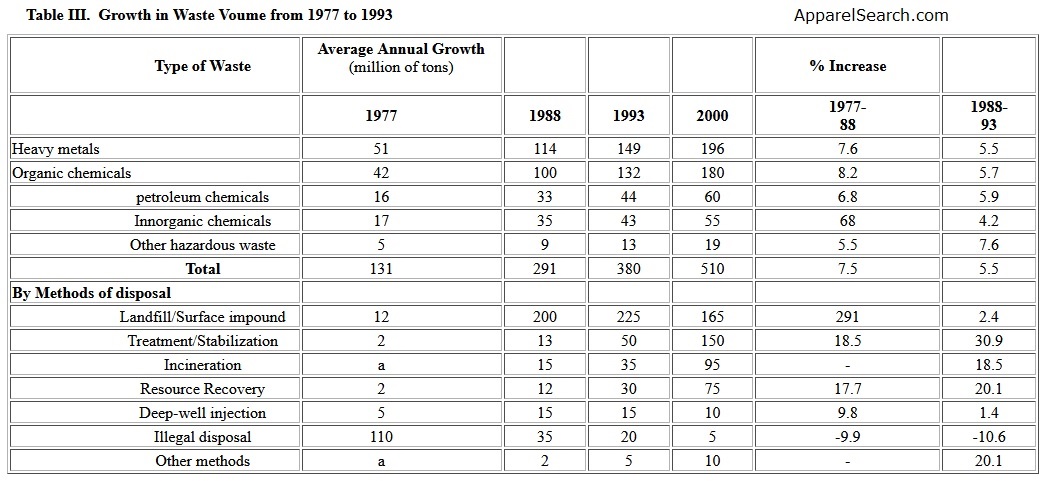
Sources of non-recyclable materials from including chemical industries, hospitals, households, generate a worldwide problem. Presently, it is estimated that about 80% of total waste remains undegradable for years or even centuries. The inappropriate disposal of these wastes on land creates the risk of contaminating ground water and causing adverse health effects. In order to properly handle the wastes, more and more polymeric geosynthetics are used as membranes or barriers. Table III gives data [2] of growth in waste volume from 1977 to 1993. The data implies that the landfills and surface impoundment will be a fact of life for many years. For this reason, geosynthetics in various forms and shapes have been developed. The requirements for geosynthetics are resistance to the environmental and chemical degradation while maintaining their physical, mechanical and chemical properties.
Table III
Biological degradation
Most synthetic Fibers are not biodegradable due to their high molecular weight and high crystallinity. The resistance is normally desirable in terms of the durability of geotextiles.
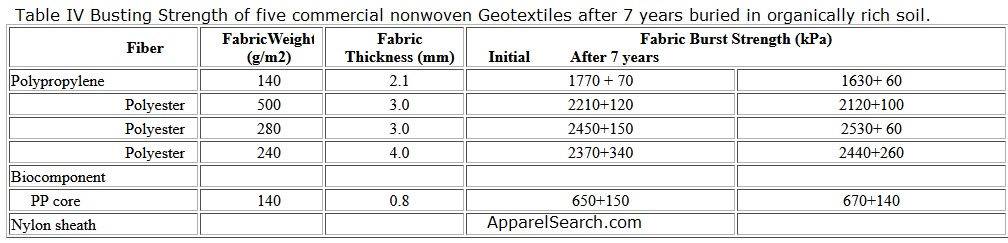
The suitable moisture and temperature in soil can promote the growth of microorganisms such as bacteria and fungi, which produce high protein substances called enzymes. Theoretically, the action of enzymes acting as catalysts can deteriorate polymer molecules. However, synthetic polymers are generally stable to microbial degradation, since they are not chemically or dimensionally compatible with the enzymes in soil. Table IV shows the busting strengths of five commercial nonwoven fabrics which were buried for seven years in an organically rich soil at a pH of 6.6 maintained at 29 oC and 85-90% relative humidity. The fabrics suffered no significant loss in their strengths during the exposure.
Table IV
In contrast, problems in solid waste disposal have led some researchers to develop new polymers that are biodegradable in order to improve the environmental impact. Poly(lactic acid) (PLA) and poly(hydroxy butyrate) (PHB) are examples. The modification of polymers such as chain branching and shorter repeat units can increase the biodegradability.
Stabilization of Polypropylene Geotextile
fiber processing exposes the polymer to high shear and often severe extrusion conditions. These conditions require a high performance stabilizer package. The high surface area-to-volume ratio of the finished fiber product requires stabilizers resistant to volatilization and extraction. Additionally, many fiber applications demand color stability through processing, aging, and exposure to NOx type gases (gas fade).
Polyolefin fiber is often stabilized through the use of a hindered phenol as primary antioxidant, in combination with a phosphite. Selection of the hindered phenol will depend on the particular performance requirements needed for each application. Issues to be considered are color development (aesthetics), thermal stability requirements, and extraction/chemical resistance.
During processing a phosphite can be used to sacrificially stabilize the polymer thus preserving the primary antioxidant for use as a long term thermal stabilizer. The structure of the phosphite can have a dramatic effect on both stabilization performance and other physical properties. Figure 4 shows the stabilizing effect on melt flow of phenol/phosphite additive packages. The large change in melt flow rate found between the compounded resin and final fiber indicates a change in molecular weight and, thus, the degree of degradation.
Figure 4 - sorry, the figure image is no longer available.
Light stabilizers
Light stability is an important issue for textiles and is particularly critical for geotextiles which may experience extended outdoor exposure. The structures of light stabilizers evaluated in this study are shown in Figure 5. Hindered Amine Light Stabilizers (HALS) outperform traditional UVAs even at one third the concentration as demonstrated in Figure 6 on outdoor weathering of natural polypropylene tapes. In TiO2 pigmented fiber, the addition of HALS results in a tenfold increase in light stability as shown in Figure 7. A concentration effect exists where stability can be increased with increasing HALS concentration.
Figure 5 sorry, the figure image is no longer available.
Figure 6 sorry, the figure image is no longer available.
Figure 7 sorry, the figure image is no longer available.
HALS are excellent stabilizers for polypropylene. HALS-1 and HALS-2 are both oligomeric; consequently, these stabilizers do not readily volatilize during processing and cannot be easily extracted. Study shows the extraction resistance of the HALS both in wash cycles at 60 oC and dry cleaning, after 5 cycles, no loss of HALS was detected by total nitrogen analysis, therefore, indicates that the HALS evaluated are fairly resistant to extraction. However, these experiments are somewhat oversimplified and extraction resistance may be dependent upon the actual exposure conditions.
HALS as thermal stabilizers
In addition to light stability, thermal stability is an important factor in polypropylene fiber stabilization. Based on the stabilization mechanism proposed for HALS, it might be expected that HALS could function as thermal stabilizers as will as light stabilizers. Indeed, when used in combination with AO-2 and PHOS-1, HALS-2 provides three times the thermal stability of a traditional high performance antioxidant AO-1 in a polypropylene tape as shown in Figure 8. Figure 9 demonstrates that HALS is an effective heat stabilizer in TiO2 pigmented polypropylene fiber at temperatures below 150 oC. The addition of HALS-2 (at 0.30%) to AO-4 increased the thermal stability by over eight-fold. HALS-1 also provides a significant benefit as a thermal stabilizer.
Figure 8 sorry, the figure image is no longer available.
Figure 9 sorry, the figure image is no longer available.
Thiosynergists
The thioether, DSTDP, has been used in combination with primary antioxidants to provide long term thermal stability. As this compound is not particularly effective at low temperatures (below 50 oC), it would not be expected to provide a significant increase in the longevity of geosynthetic materials. At lower test temperatures, HALS provide significantly higher stability than systems containing thiosynergists. In fact, when HALS are combined with thioethers the thermal stability is lower than for systems containing HALS alone.
Carbon Black Filled Systems
Many geotextile applications require gray or black pigmentation. Although carbon black is often included to increase light stability, it typically has a negative effect on thermal stability. As carbon black levels increase, the thermal stability provided by the base antioxidant system decreases as shown in Figure 10. It is believed that the large surface area of the carbon black is capable of adsorbing antioxidants, rendering them ineffective. Apparently, polymeric HALS such as HALS-2 are not as adversely effected by carbon black and can provide a dramatic increase in heat stability.
Figure 10 sorry, the figure image is no longer available.
Traditionally, carbon black alone was believed to provide sufficient light stability. Xenon weatherometer testing on polypropylene tapes has shown that if a HALS is added to carbon black filled polypropylene, carbon black concentrations can be lowered to one quarter their original level and better light stability will still result, as shown in Figure 11. As discussed above, reducing the carbon black level has the additional benefit of enhancing other desirable properties. HALS-1 shows better light stability performance than HALS-2. This might be attributed to greater steric hindrance of the tertiary amine (HALS-1) which may reduce its interaction with the carbon black surface.
Figure 11 sorry, the figure image is no longer available.
In practice, various mixtures and combinations are used depending on the desired performances. For example, in application in hydraulic constructions, the stabilizers with high molecular weights are required to avoid washing out. Also different types of stabilizers may have different effects at different temperatures.
Resistance to chemicals and living-organisms
Due to its hydrocarbon chemical structure, PP is extremely stable to most of the organic and inorganic chemicals such as acids, alkalis, inorganic salts, detergents, oils and gasoline. PP is vulnerable only to substances like peroxide, concentrated acids, halogens and some chlorinated or aromatic hydrocarbons. In addition PP is highly stable against hydrolysis, ammonalysis, and the majority of chemical substances found at disposal sites.
In the terms of its biological stability, PP is extremely stable against fungi and bacteria. Even though the growth of microorganisms may be found on the surface, it will not affect the mechanical properties of PP. Since PP cannot be digested by insects, it also has moth-proof properties.
Other Factors
Other factors to be considered include processing variables due to
dependence of fiber properties on them, such as the degree of orientation
and fiber fineness. Additives, such as pigments on UV stabilizers, also
affect durability of geotextiles. Table V and VI show that the high draw
ratio and the high fineness of PP and the brown pigments enhance the
stabilizing processes. In conclusion, the oxidative stability of PP are
determined by the following factors:
- the type and purity of PP polymer
- manufacturing processes - temperature and drawing ratio
- stabilizing system
- environmental factors.
Table V & VI sorry, the table image is no longer available.
V. Polyesters
By the modification of manufacturing processes, the great versatility of polyesters allows production of a broad range of products such as moderate strength and high elongation. The physical properties of polyesters, such as PET, have excellent toughness and dimensional stability. Also, its durability to moisture and microorganisms is excellent. In this section, the aging mechanisms of PET such as thermo- and photo- degradation, and resistance to chemicals, especially hydrolysis, will be discussed.
Resistance to Photo- and Thermo-degradation
Generally speaking, polyesters have higher resistance to sunlight than
does polypropylene. Figure 12[15] shows the tensile strength change of PET
and PP after 9 months outdoor exposure. Considering that there is the
possibility that hydrolysis degradation occurs in the same time, tensile
strength of PET still decreases slower than PP does. Even though untreated
PET may be slowly degraded by UV radiation, long term UV degradation slows
down and can level out, as shown in Figure 13. Also, added stabilizers can
improve resistance to photo-degradation. PET is only sensitive to UV
between 3000 to 3300
, which is very easily blocked. The outer layers of
the fabric itself can filter this radiation.
Figure 12 sorry, the figure image is no longer available.
Figure 13 sorry, the figure image is no longer available.
PET has outstanding thermal properties. Below freezing and at temporarily high temperatures, its strength retention is excellent. Even at temperatures up to 400 oF, more than half the strength is retained over several days. Figure 14 shows the excellent thermal resistance of PET-well above normal conditions encountered in geotextile applications.
Figure 14 sorry, the table image is no longer available.
Resistance to Chemicals
Generally PET has excellent resistance to chemicals such as water, salts, organic acids, organic solvents, oxidizing and reducing agents, gases, and oils. But it is vulnerable to some chemical classes under certain conditions (Table VII). PET is highly resistant to most acids such as nitric and sulfuric acids, but some acids such as chlorosulfonic acid can destroy PET immediately (Table VIII). Therefore, in order to avoid acid catalyzed hydrolysis, the environmental conditions must be known before applying PET geotextiles.
Table VII, VIII and IX - sorry, the table image is no longer available.
In the case of alkali resistance, PET has limited resistance as shown in Table IX. Even though its degradation can not be generalized, the mechanisms can be categorized two ways:
- alkaline hydrolysis by hydroxide nucleophiles attacking ester groups
- nucleophilic amidation by amines
In most cases, PET are very stable against inorganic and organic chemicals. However, some classes such as ammonium sulfide and alkyl amines can degrade PET severely. In conclusion, to avoid acid and alkaline hydrolysis, and other chemical degradation, each environment of PET application area should be examined.
Conclusion
Most of the general effects on the durability of geotextiles and degradation mechanisms have been discussed. Aspects for prevention of degradation of PP and PET geotextile have been suggested. Effective stabilization of polypropylene and polyester Fibers for geotextile applications required an additive system capable of preventing polymer degradation during both processing and aging. In general, all possible factors should by considered and studied before selecting the geotextile fabric for a given application. Weathering and soil conditions including time, temperature, and chemicals can be prior considerations. Most synthetics are good raw materials for geotextile applications because the nature of synthetic polymers are very resistant to light, chemicals and living organisms. However, under certain conditions, they can be degraded. The main concern for PP geotextiles is to prevent thermo- and photo-degradation by using proper stabilizers. On the other hand, PET geotextiles should be applied in certain environments where acid and alkaline hydrolysis condition can be avoided. Therefore, the durability of geotextiles depends on the proper selection of raw materials.
References
- M. L. Marienfeld Tappi Journal 78 (9) pp 143-146 (1995)
- J. D. Ortego, T. M. Aminabhavi, S. F. Harlapur, and R. H. Blundgi
Journal of Hazardous Materials 42 pp 115-156 (1995)
- R. Fried, Polymer Science and Technology, Prentice Hall PTR, New
Jersey 1995
- Tisinger, L. G., Geotechnical Fabrisc Report, 7(2), pp 22 (1989)
- Ramaswamy, S. D., Rathor, M. N., Proc. fourth International
Conference on Polypropylene Fibers and Textiles, Nottingham, UK,
September, 1987
- Y. A. Shlyapnilov, S. G. kiryushkin and A. P. Mar'in, Antioxidative
Stabilization of Polymer. Taylor & Francis, Printed in Great Britain by
T. J. Press Ltd, Cornwall, 1996
- Bolland, J. L., Gee, G., Trans, Faraday Soc., 42, pp 236-244, (1946)
- G. Wypych Handbood of Material Weathering ChemTec Publishing, Canada
(1995)
- Das, P.K., Encinas, M. V., J. Am, Chem Soc., 103, pp 4162 (1981)
- Pospisil, J., Adv, in Polym. Sci., 36, pp 69 (1980)
- Denny, D. B., Goodyear, E. F., J. AM. Chem. Soc., 82, pp 1393 (1960)
- Zuech, E. A., Chem. Comm., pp 1182 (1968)
- Chang, B. C., Denny, D. B., J. Am. Chem. Soc., 91, pp 5243 (1969)
- Denny, D. B., Jones, D.H., J. Am. Chem. Soc., 91, pp 58211 (1969)
- E.W.Brand, Fellow, ASCE, and P. L. Richard pang Journal of
Geotechnical Engineering, 117(7) pp 979-1000 (1991)
- R.M. Koerner and J. P. Welsh, Construction and Geotechnical
Engineering Using Synthetic fabrics, John Wiley and Sons, New York,
(1980)
- All other source information is obtained from original author Hageun Suh and Ping Hao
http://trcs.he.utk.edu/textile/nonwovens/durability.html
Apparel Search
Add Your Company Contact
Us About Us Advertise
News Letter Legal
Help
Copyright © 1999-2023 Apparel Search Company.
All Rights Reserved.