Chemical Bonding (Educational Research)
Monika Kannadaguli & Ramaiah Kotra
Introduction
For more than four decades, almost all nonwovens required a chemical binder in order to provide any measure of structural integrity. In addition, the binder was called upon to contribute and convey numerous properties that were necessary for the effective performance of the fabric.
During this extended period, binders were essentially the weak element in developing fully acceptable nonwoven fabrics. The Fibers that were available to the nonwoven industry were the same Fibers that were available to the textile and other fiber-based industries; hence, the Fibers were fully acceptable. It was generally the binder that limited the performance of the nonwoven fabric.
The deficiencies cited against nonwovens generally were deficiencies attributable to an inadequate binder. Common complaints are as follows:
- The fabric doesn't have enough strength.
- The fabric is too stiff.
- The fabric has inadequate absorbency.
- The fabric shows poor launderability.
- The fabric has inadequate dry cleanability.
- The fabric simply doesn't feel like a textile.
As a consequence, a great deal of effort has been put into the development and continuous improvement of chemical binders. The steady improvements in nonwovens performance that occurred over a period of many years were, in no small measure, due to improvements in the performance and utility of the binder.
In the very early stages of nonwovens development, different types of natural resins and glues were used to bond nonwovens. While they conveyed some integrity and strength to these webs, they also had many glaring deficiencies. Consequently, synthetic binders were developed to meet the structural and performance requirements of nonwoven fabrics.
Polyvinyl acetate was the first successful synthetic binder used in substantial volume. This material had distinctly superior adhesive properties, strength, and performance compared to the early natural adhesives. This binder had considerable flexibility in formulation, and could easily be applied to fiber webs by a variety of application processes, including print bonding.
The industry was faced with the inevitable compromise in fabric properties of nonwovens bonded with synthetic materials. In order to build strength in the fabric, increasing amounts of resin must be applied, which results in more stiffness. If softness is necessary, it can be achieved, but primarily by sacrificing strength.
A substantial improvement in this trade-off of strength and softness was achieved with the introduction of acrylic-based latex binders in the 1950s and 1960s. By proper selection of co-monomers, it is possible to build improved softness properties with adequate strength. Consequently, these binders became widely used by most of the nonwovens industry, despite the somewhat higher cost.
As polymer technology for manufacturers of synthetic binder systems improved, a greater variety of chemical building blocks became available with much greater flexibility in terms of binder strength, durability, and other properties. The introduction of cross-linkable and self-crosslinking binder polymers turned out an entirely new range of fabric properties. This was particularly noteworthy in durable nonwovens where such durability features as washability and dry cleanability were important.
Requirements
The construction of a nonwoven with suitable binders is to achieve improved characteristics such as strength, softness, adhesion, firmness, durability, stiffness, fire retardence, hydrophilicity, hydrophobicity, anti-microbial properties, organic compatibility, reduced surface tension, improved dimensional stability and solvent, wash and acid resistance. The following list illustrates some general considerations required for an ideal binder. The required properties can be varied depending on the end-uses.
- Strength:
Classification of Binders
Due to their diversity binders may be classified into several categories based on polymer (binder) chemical structure, functionality and the type of curing reactions.
1. Classification based on chemical structure.
There are three main kinds of binders: butadiene copolymers, acrylates, and vinyl copolymers. The chemical compositions influence Tg, hardness and softness, hydrophobicity and hydrophilicity, elasticity, aging, and dry tensile strength of binders. The higher the Tg, the stiffer the hand and higher the dry tensile strength of binders.
Butadiene Copolymers
The structures of two main butadiene copolymers are shown as follows:
| Styrene/butadiene rubber (SBR) |
| -(-CH2-CH=CH-CH
2-CH-Ch2-)n- f where f denotes a phenyl group. |
| Butadiene/acylonitrile Rubber (NBR) |
| -(CH2-CH=CH-CH2-CH-CH2-)n- CN |
The butadiene polymers are cross-linked by polysulphides, and their properties are modified by different copolymers. The butadiene monomers provide elasticity while styrene and acrylonitrile monomers give tensile strength, and oil and solvent resistance, respectively. Their disadvantages are oxidation and discoloration due to residual double bonds in their polymer chains.
Acrylic acid derivatives
Acrylic binders are the most widely used and versatile binders available with various modifications. The properties of acrylic binders differ according to their derivatives and copolymers. The structures of the common acrylic polymers are as follows:
| Acrylic Acid Derivatives |
| Acrylic Acid: -CH2-CH- COOH __________________________________ Acrylates: -CH2-CH- COOR _____________________________________ Acrylamide: -CH2-Ch- O=C=NH2 ___________________________________________________
Metholyated Acrylaminde: -CH2-CH-
O=C-NHCH2-OH ________________________________ |
They are frequently copolymerized with styrene, acrylonitrile, vinyl chloride or vinyl acetate, depending on the desirable properties. Some of these properties are hardness from styrene, solvent resistance from acrylonitrile, flame retardancy from vinyl chloride, and cost benefits from vinyl acetate.
Vinyl copolymers
There are two main binders for vinyl copolymers: vinyl
chloride and vinyl acetate. Since the vinyl binders are stiff, they are
plasticized externally or internally. As internal plasticizers, ethylene
and acrylate monomers are used, and external plasticizers consist of vinyl
chloride. Due to its low Tg, vinyl acetate is not that stiff, and its
advantage is low cost. The chlorides cause yellowing problems. The
chemical structures are closely related Tg and stiffness of binders. The
Tg's of many homopolymers are listed in
Figure 1
2. Classification based on functionality
The functionality of binders is in the functional groups attached to polymer chains, which influences wet and solvent properties. To modify binder properties, copolymerization with a small amount of monomers with special functionality is performed. The main functionalities in binders are carboxyl and amide side chains.
Carboxyl functionality
This functionality is related to binders which contain acrylic acid or methacrylic acid by copolymerization. The binders are crosslinkable since the functional group, carboxylic acid, provides sites for crosslinking reactions. Two probable cross-linking reactions of carboxylated lattices are shown as follows:
| Metal oxides: |
| -CH2-CH- -CH2-CH- COOH Heat C=O -------------- COOH +ZnO Zn + H20
-CH2-CH- C=O -CH2-CH |
| Amino resins: |
| -CH2-CH- -CH2-CH- COOH CO=O
CH2 NH CH2-NH
R -------------------> R +H20
CH2 NH CH2-NH
COOH C=O -CH2-CH- -CH2-CH- |
Amide functionality
This functionality is related to binders containing acrylamide by copolymerization. The amide functionality provides crosslinking sites, and even the binders are self-crosslinkable .
N-metylol amide functionality
This functionality is obtained after acrylamide is reacted with formaldehyde. The binders containing the substituted acrylamide groups have self-crosslinking properties, and the possible reaction as follows:
| Acrylaminde: |
| -CH2-CH- -CH2-CH- O=C=NH2 Heat O=C -----------------> NH O=C-NH2 -NH3 O=C -CH2-CH2- -CH2-CH- |
| Substituted acrylamide |
| -CH2-CH- -CH2-CH-
O=C O=C
NH NH
CH2OH ------------------> CH2 CH2OH NH
NH O=C
O=C -CH2-CH- -CH2-CH- |
3.Classification based on type of curing reactions.
The classification of reactivity refers to crosslinkability of binders, which is related to reaction with curing resins, crosslinking agents. The most common curing resin is melamine formaldehyde condensate resin which involves reaction of n-methylol groups.
Non-crosslinkable polymers
The polymers do not contain any of the functional groups. They can not crosslink , even with external curing resins.
Crosslinkable polymers
The polymers contain acid or amide functional groups. They can react with added curing resins, but the degree of crosslinking is limited.
Self-crosslinking polymers
The polymers contain n-methylol functional groups. They can react with themselves, and a high crosslink density can be obtained by adding curing resins.
Recent trends in chemical bonding: Although nonwoven manufacturers are seeking alternative technologies such as thermal bonding, chemical bonding still has its advantages and a promising market. Chemical bonding allows more room for fabric designs and fiber selections. Both disposable and durable products are supplied to roll goods producers and fiber manufacturers. On the environmental front, increasingly strict regulations and guidelines are driving a trend towards alternative products and technologies. Manufacturers and end-product suppliers alike are seeking ultra-low or formaldehyde-free binders. The growing consideration of the environmental impact of chemical binder and additives has become a focus of debate on the national and international level.
Latex binder chemical types
A latex polymer consists of an aqueous medium with extremely fine liquid or solid polymer particles dispersed therein. The latex polymer generally is produced via free radical emulsion polymerization in water, whereby a vinyl monomer is combined with a small amount of other monomer (co-monomer) to create a high molecular weight polymer. The latex dispersion also will carry surfactants, stabilizers, and other additives to convey realistic properties to the latex itself.
When used as a binder, the latex typically is combined with other components to provide the formulated binder ready for application to the fiber web. The formulated binder conveys many characteristics that are not possessed by the straight binder. Consequently, there is a substantial chemistry involved in combining the latex with the other components in order to prepare the formulated binder.
Binders are quite dependent upon the glass transition temperature (Tg) of the monomer unit selected to form the polymer. The lower the (Tg) of the monomer units, the softer is the resulting polymer. A sampling of the most common monomers used in the manufacture of latex polymer for nonwoven binders include the following materials:
monomer Tg(0C)
Ethylene -125
Butadiene -78
Butyl Acrylate -52
Ethyl Acrylate -22
Vinyl Acetate +30
Vinyl Chloride +80
Methyl Methacrylate +105
Styrene +105
Acrylonitrile +130
The monomers selected for forming the polymeric latex also have considerable influence on the hydrophilic or hydrophobic nature of the binder. This can affect the wet strength of the nonwoven fabric as well as a host of absorbency characteristics.
With the current capabilities of polymerization chemistry, there is considerable versatility for each chemical type. Despite this range of properties, the commonly employed nonwovens binders generally are characterized by a fairly well defined set of properties. These properties can be modified to some degree by incorporation of other agents, but they provide a useful guide in classifying the kind of performance to be expected from each type of binder.
Types of nonwoven binders
The following comparison of latex binder chemical types provides an indication of the relative performance, as well as the advantages and disadvantages of each type of binder. As indicated, the binder properties can be modified considerably by the presence of co-monomers.
Acrylic: These binders offer the greatest durability, color stability, and dry/wet performance. Acrylic binders have the widest range of fabric hand properties. They can be formulated to vary from very soft (Tg = -40oC) to extremely hard (Tg = 105oC). These binders can be used in virtually all nonwovens applications, although they tend to be more costly. These polymers can be made to cross-link, with substantial improvement in durability.
Styrenated Acrylics:
These are tough, hydrophobic
binders. The resulting textile hand ranges from soft-to-firm (Tg varies
from
20oC to +105oC ).These binders can be used in
applications where there is a need for some wet strength without
crosslinking. The use of this type of latex binder does involve some
sacrifice in UV and solvent resistance.
Vinyl Acetate (VAC): The vinyl acetate binders are firm (Tg = +30oC to +40oC); however, they are relatively low cost and find extensive use. They offer good dry strength and toughness, but are somewhat hydrophilic and have a tendency to yellow when subjected to heat.
Vinyl Acrylics: These binders are more hydrophobic than the straight VAC binders. They provide excellent toughness, flexibility, and better color stability. They are the compromise between VAC and acrylic, and can compete on a cost/performance basis. The hand range is limited to intermediate softness (Tg = -10oC) to a firm hand (Tg = +30oC).
Ethylene Vinyl Acetate (EVA):
These latex binders
have a (Tg range of
20oC to +115oC, which is
equivalent to soft ranging to an intermediate textile hand. They exhibit
high wet strength, coupled with excellent absorbency. In general, they are
less costly than acrylics. They do have a tendency to have more of an odor
compared to other binders. They are used primarily in wipes, air-laid pulp
fabrics and similar applications.
Styrene-Butadiene (S/b, SB, or styrene butadiene rubber): These binders have an excellent combination of flexibility and toughness. They range in hardness from very soft (Tg = -30oC) to very firm (Tg = +80oC). However, the (Tg of an SB binder is not strictly comparable to other classes of nonwoven binders. The styrene-to-butadiene ratio (S/b ratio) is the most common method for describing the relative hand resulting from the use of these binders. The higher the styrene content, the firmer the hand. When cross-linked, this class of binder is very hydrophobic and durable. They are affected somewhat by heat and light because of their tendency to oxidize.
Polyvinyl Chloride (PVC): The homopolymer of polyvinyl chloride is a very hard, rigid polymer (Tg = +80oC). This polymer must be plasticized to provide flexibility and film-forming properties. Normally, the (PVC) binders used in nonwovens are softened internally by co-polymerizing the vinyl chloride or with softer acrylic monomers. The hand range of most of these polymers is still relatively firm (Tg is greater than the +30oC). Because this type of polymer is quite thermoplastic, it performs well in heat and dielectric sealing applications. This can be an advantage in some uses. Also, the chlorine content of the polymer promotes flame retardancy. This feature is one of the primary benefits of utilizing this type of binder. However, the chlorine also conveys the tendency to yellow upon heat aging, due to elimination of hydrogen chloride from the polymer.
Ethylene/vinyl Chloride (EVCI): Binders in this class have a slightly broader hand range (Tg = OoC to +30oC) without the external plasticization required of (PVC) binders. The presence of the chlorine again conveys some flame retardancy. These binders exhibit good acid resistance, fair water resistance, and excellent adhesion to synthetic Fibers. There is some tendency to yellow upon aging. In essence, this is an internally plasticized (PVC) binder, considering the ethylene monomer to be the softener.
FORMULATION
Ingredients
The formulation of binding solution is an art since many ingredients are involved and many different possibilities exist for different end-uses. Some of the characteristics, and the types of formulation agents utilized to obtain them include the following.
- Surfactants : offer improvement in binder adhesion, stability, and ability to be converted into a foam
- External cross-linkers: provide cross-links with binder polymer to provide improved performanc
- Defoamers: utilized to minimize foam in processing
- Repellent agents : convey water or oil repellency
- Salts: added to impart low flame response properties and to convey antistatic properties
- Thickeners: added to control the rheology of the binder liquid
- Catalysts: added to facilitate curing and to promote cross-linking
- Acids and bases: added to control pH of the latex
- Dyes and pigments: provide color to the binder and fabric
- Fillers: added to reduce binder tack and to lower cost
- Optical brighteners: added to increase whiteness
- Sewing aids: added to provide lubrication during fabrication
The purposes of wetting agents, mainly nonionic or anionic surfactants, are to enhance binder penetration through webs, improve the affinity between binder and Fibers. The crosslinker, which has multi-functional groups, is generally added to increase crosslink density and to improve durability and resistance to deformation. The typical reaction of the common crosslinking agent, melamine formaldehyde, is illustrated as follows:
| Melamine formaldehyde: |
| -CH2-CH- -CH2-CH-
O=C O=C
NH NH
H OH----CH2 H2C----OH H
NH NH N
C C
N N C O=C NH NH
H2C |
Order of Formulation
In terms of adding ingredients into a binding bath, the compatibility of ingredients should be confirmed because the orders are extremely important . The milky white color of most binders impedes a check on the white-color indication of non-compatible ingredients, so most ingredients are first added to the dilution water. After the compatibility is assured, binders are added and then thickeners added to adjust viscosity. For the stability of the binding solution, catalysts are added just before application. Some water may be added to reach a desirable solid level. The summarized order is as follows:
Most ingredients
Latex binder
Thickener
Catalyst
Some water, and the others, such as dyes and pigments, fillers, clays, optical brighteners, sewing aids, etc.
BONDING TECHNOLOGY
Bonding nonwoven webs
Web consolidation or nonwoven bonding processes interlock preferentially arranged fiber or film assemblies by mechanical, chemical, solvent, and/or thermal means. The degree of bonding is a primary factor in determining fabric integrity (strength), porosity flexibility, softness, and density (loft, thickness).
Bonding may be carried out as a separate and distinct operation, but generally is carried out as a sequential operation in tandem with web formation. In some fabric constructions, more than one bonding process may be used to enhance physical or chemical properties.
Mechanical consolidation methods include needlefelting, stitchbonding, and hydroentangling. Chemical consolidation methods involve applying adhesive binders to webs by saturating, spraying, printing, or foaming techniques. Solvent bonding involves softening or partially dissolving Fibers with a solvent to provide self-bonding surfaces. Thermal bonding involves the use of heat and often pressure to fuse or weld Fibers together at points of intersection or in patterned bond sites.
Important issues to consider when choosing the web consolidation method are economy; versatility; and product properties, primarily absorbency, strength, softness, loft, and purity. A recurring issue involves environmental requirements of both the process and the product. Many techniques are done for specific properties of unique fabrics; therefore, it is difficult to measure differences in cost. In some instances, two or more bonding techniques compete. The system that is most energy-efficient; environmentally sound or provides the preferred fabric properties generally dominates.
CHEMICAL BONDING PROCESSES
Chemical or resin bonding is a generic term for interlocking Fibers by the application of a chemical binder. The chemical binder most frequently used to consolidate fiber webs today is a water-borne latex. Most latex binders are made from vinyl materials, such as polyvinylacetate, polyvinylchloride, styrene/butadiene resin, butadiene, and polyacrylic, or their combinations.
Latexes are extensively used as nonwoven binders, because they are economical, versatile, easily applied, and effective adhesives. The versatility of a chemical binder system can be indicated by enumerating a few factors which are considered when such a system is formulated.
The chemical composition of the monomer or backbone material determines stiffness/softness properties, strength, water affinity (hydrophilic/hydrophobic balance), elasticity, durability, and aging. The type and nature of functional side groups determines solvent resistance, adhesive characteristics, and cross-linking nature. The type and quantity of surfactant used influences the polymerization process, polymer stability, and the application method.
Chemical binders are applied to webs in amounts ranging from about 5% to as much as 60% by weight. In some instances, when clays or other weighty additives are included, add-on levels can approach or even exceed the weight of the web. Waterborne binders are applied by spray, saturation, print, and foam methods. A general objective of each method is to apply the binder material in a manner sufficient to interlock the Fibers and provide fabric properties required of the intended fabric usage.
The common methods of bonding include saturation, foam, spray, print and powder bonding. They are briefly introduced in the following paragraphs:
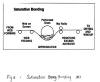
1.Saturation
Saturation bonding is used in conjunction with processes which require rapid binder addition, such as card-bond systems, and for fabric applications which require strength, stiffness, and maximum fiber encapsulation, such as carrier fabrics. fiber encapsulation is achieved by totally immersing the web in a binder bath or by flooding the web as it enters the nip point of a set of pressure rolls. Excess binder is removed by vacuum or roll pressure.
Three variations of saturation bonding exist: screen, dip/squeeze, and size-press. Screen saturation is used for medium-weight nonwovens, such as interlinings. Dip/squeeze saturation is used for web structures with strength sufficient to withstand immersion without support, such as spunbonds . Size-press saturation is used in highspeed processes, such as wet-laid nonwovens. Drying and curing may be carried out on steam-heated drying cans or in thru-air ovens or perforated-drum dryers. Binder addition levels range from 20% to 60%. Two techniques, single screen saturator and applicator roll technique, are illustrated in Figure 2 and Figure 3. Advantages of this method are simplicity, controllable tensile strength and softness by choice and amount of binders. The disadvantages are the great influence of binders on softness, and the limitation in loftiness.
Figure 2
2.Foam bonding
Foam bonding is a means to apply binder at low water and high binder-solids concentration levels. The basic concept employed involves using air as well as water as the binder diluent and carrier medium. Foam-bonded nonwovens require less energy in drying, since less water is used. The foam is generated by introducing air into the formulated latex while mechanically agitating the binder solution.
Air/latex dilutions or blow ratios in the order of 5:25 are practiced for various products. With the addition of a stabilizing agent to the binder solution, the foam can resist collapsing during application and curing, and the bonded fabric will exhibit enhanced loft, hand, and resilience. Non-stabilized foams are referred to as froths; froth-bonded fabrics are similar in properties to some saturation-bonded nonwovens. One example of this bonding is illustrated in Figure 4 The advantages include less energy required to dry the web, less binder migration and controllable softness by choices and amount of binders. The disadvantages are difficulties in controlling process and adequate foaming.
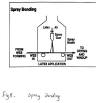
3.Spray bonding
In spray bonding, binders are sprayed onto moving webs. Spray bonding is used for fabric applications which require the maintenance of highloft or bulk, such as fiberfill and air-laid pulp wipes. The binder is atomized by air pressure, hydraulic pressure, or centrifugal force and is applied to the upper surfaces of the web in fine droplet form through a system of nozzles.
Lower-web-surface binder addition is accomplished by
reversing web direction on a second conveyor and passing the web under a
second spray station. After each spraying, the web is passed through a
heating zone to remove water, and the binder is cured (set/cross-linked)
in a third heating zone.
For uniform binder distribution, spray
nozzles are carefully engineered. Typical spray bonding is illustrated in
Figure 5
4.Print bonding
Print bonding applies binder in predetermined areas only and is used for fabric applications that require some areas of the fabric to be binder-free, such as wipes and coverstocks. Many lightweight nonwovens are print bonded. Printing patterns are designed to enhance strength, fluid transport, softness, hand, absorbency, and drape. Print bonding is most often carded out with gravure rolls. Binder addition levels are dependent on engraved area and depth as well as binder-solids level. Increased pattern versatility can be achieved with the use of rotary screen rolls. Drying and curing are carried out on heated drums or steam-heated cans.
In print bonding, high viscose binders are applied to limited, patterned areas. A prewet/prebond step is required for enough strength of webs, and typical steps in this bonding are in Figure 6 There are two types of printers: rotary screen and rotogravure printers. Binders are applied through a hollow applicator roll in rotary screen printer, while in rotogravure printer they are applied by an engraved applicator roll as shown in Figure 7 The main advantage is that outstanding softness of nonwoven fabrics with adequate strength can be achieved.
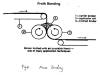
5.Powder bonding
In powder bonding, the adhesive powder of thermoplastic polymers is applied onto webs by heat and pressure. Polyesters and polyolefins with low Tg's and molecular weight can be used as powder binders. A typical bonding line is illustrated in Figure 8 The advantages are the bulky structure of dense nonwovens and the applicability of polyester or polypropylene webs. The disadvantage lies in difficulties of suitable particle sizes and ranges, and their distribution.
APPLICATIONS
The following list provides an indication of those nonwoven end-use applications in which binder-bonded nonwovens are utilized:
1. Typical nonwoven applications:
- Wipes and towels
- Medical nonwovens
- Roofing products
- Apparel interlinings
- Filter media
- Coating substrates
- Automotive trim
- Carrier fabrics
2. Typical highloft nonwovens:
- Bedding products
- Furniture applications
- Apparel
- Filtration media
- Pillows
- Automotive trim
SUMMARY
In the latter part of the 1970s and 1980s, thermal bonding technology grew rapidly, providing the industry with a realistic method to produce strong and soft nonwoven fabrics without the use of a chemical binder. This development provided substantial advances in performance and properties of many types of nonwovens. One quality of this new bonding technique was that these nonwovens contained no formaldehyde and no chemical additives to cause consumer concern. Naturally, this movement depressed the interest of chemical binders within the industry and has resulted in a decline in binder usage.
Despite this setback, significant improvements and advances have continued to be made by the synthetic polymer industry, to the benefit of the range of nonwoven products that continue to utilize chemical bonding methods. These improvements have involved such developments as formaldehyde-free binders, low-cure temperature binders, complex copolymers with unique characteristics, moldable binders, and others.. In the future, new types of binders may be combined with the present choices, for example, by copolymerization. Also, new bonding technology may occur. In addition, new ideas such as reactive binders which can be covalently bonded with Fibers, will be continually investigated.
References
- W.E. Devry, "Latex bonding Chemistry and Processes."
- A. Derelich, "Nonwoven Textile fabrics", Kirk-Othmer:
Encyclopedia of Chemical Technology, Vol. 16, 3rd Ed, p104-124,
1981.
- B.M. Lichstein, The Nonwovens Handbook, Inda Association of the
Nonwoven fabric Industry, New York, 1988.
- J. Lunenschloss and W. Albrecht, Non-woven Bonded fabrics, John
Wiley & Sons Inc., New York, 1985.
- A.E. Meazey, " Binders used in Bonded fiber fabric Production",
Nonwovens '71, The textile Trade Press, England, 1971.
- M.F. Meyer and W. A.; Haili, " Nonwovens and Laminates Made with
Polyester Adhesive Powders, "Eastman Kodak Company.
- J.M. Oelkers and E.J. Sweeney," Latex Binders and Bonding
Techniques of Disposables", 1988.
- Ellen Lees Wuagneux, " And how would you like your nonwovens?"
Nonwoven Industry Oct. 64-72 (1997).
- INDA, Book of Paper, 1997
- http://www.nonwovens.com/facts/technology/binders/binders.htm
Visit our nonwoven education page.
Apparel Search
Add Your Company Contact
Us About Us Advertise
News Letter Legal
Help
Copyright © 1999-2023 Apparel Search Company.
All Rights Reserved.
Buy Fashion
For The Holidays.